Themes > Features
03.04.2001
Patents and the Multinational Drug Industry
It has been suspected,
or even known, for some time now that multinational drug companies not
only make very high profits through charging monopoly prices on various
drugs, but also use national and international patent regimes to further
strengthen such monopoly. However, even the fiercest critics of the
TRIPS agreement may not have anticipated the extent of such practice
even in the case of life-saving drugs, as has been revealed in the recent
controversy over the sale of AIDS drugs in Africa.
The controversy involves a Mumbai-based Indian drug company, Cipla Limited
and the multinational drug giant Glaxo Wellcome plc. Cipla has become
one of the world's major producers of generic AIDS medicines, based
on the company's ability to produce drugs through reverse engineering.
This in turn is possible because the Indian patent laws still recognise
only process patents in the pharmaceutical sector.
Late last year, Glaxo attempted to block access to cheaper versions
of its top-selling AIDS medicine which were being distributed in Sub-Saharan
Africa by Cipla. In early 2000, Healthcare Ltd., a pharmaceutical distributor
in Accra, Ghana, purchased a small consignment of Duovir, Cipla's version
of Glaxo's anti-AIDS drug Combivir. Cipla was providing these at a small
fraction of the cost charged by Glaxo. Soon afterward, Glaxo sent letters
to Cipla and Healthcare charging that "importation of Duovir into
Ghana by Cipla or its affiliates represents an infringement of our company's
exclusive patent rights" and threatening legal action if they were
continued. As a result, Cipla stopped selling Duovir in Ghana. Healthcare,
the Ghana distributor, said boxes of Duovir remain unopened in its offices
and that no patients have received any of the drug, in a country with
one of the most severe problems of AIDS incidence.
Currently, the cocktail of three drugs that are used to treat AIDS patients
is provided by the major MNC drug companies to developed country users
at a price of around $10,000 a year. This an amount which is obviously
outside the reach of most African citizens. Yet it is estimated that
of the total of 36 million people in the world currently infected with
AIDS, as many as 25 million are in Sub-Saharan Africa. Cipla, which
manufactures generic versions of these drugs, has offered them for sale
to several South African countries at just above $ 300 a year. Cipla
has been cutting prices of these drugs continuously over the past year,
citing in-house technological advances as the cause.
It is noteworthy that over the past year, five major drug makers - Glaxo,
Bristol-Myers Squibb Co., Merck & Co., Boehringer Ingelheim GmbH
of Germany and Roche Holding Ltd. of Switzerland - have agreed to substantially
slash prices of their AIDS drugs in Africa, even though they are still
much above the price of the generic substitutes. It is known that this
offer to discount prices is largely because of the fear that African
nations will begin buying generic copies of their drugs produced by
Cipla in India and by other companies in Thailand and Brazil.
In a recent twist, Cipla has offered to pay a royalty of 5 per cent
of sales to the five drug companies, a move which has met with tepid
response. Instead, the MNCs have argued that all this condones the violation
of the companies' patents and the TRIPS agreement, and have asked western
governments to put more pressure on India, Brazil and other countries
to speedily adjust their patent laws so as to conform to TRIPS and prevent
such production of cheaper generic drugs, in other words to regain their
monopoly and ability to charge higher prices.
This story, concerned as it is with life-saving drugs, brings into stark
relief the nature of the international pharmaceutical industry and the
implications of the TRIPS agreement for basic public health. Conversely,
the multinational pharmaceutical companies argue that without intellectual-property
protection they would have no incentive to invest the millions required
to discover and develop new drugs. The irony in this case is that the
costs of developing the two chemicals (AZT and 3TC) used in Glaxo's
Combivir were actually borne by the public sector - through work done
earlier by researchers funded by the US Government's National Institute
of Health. Glaxo purchased the drug before its efficacy in AIDS treatment
became obvious, and once again relied on public research to establish
this efficacy. However, once it was known, the company lost no time
in taking out a patent on this drug and reaping monopoly profits from
its sale.
Pharmaceutical markets differ from markets for most other commodities,
since drugs are a rather special commodity. Private drug markets typically
suffer from a number of forms of market failure, including (a) informational
imbalances; (b) monopoly and lack of competition created by patent protection,
brand loyalty and market segmentation; (c) externalities in the form
of social benefits of drug consumption. In addition, of course, there
are obvious welfare implications, since unregulated drug markets tend
to create substantial inequity, particularly in terms of access to drugs.
For these reasons, there have been major concerns about the enforcement
of the TRIPS agreement particularly with reference to health conditions
in developing countries. Some of the most frequently expressed concerns
have included the following :
(i) Increased patent protection will lead to higher drug prices, while
the number of patented drugs of importance from a public-health point
of view will increase in the coming years.
(ii) The access gap between developed and developing countries, and
between rich and poor in all countries, will increase, especially as
producers in developing countries would have to wait for 20 years before
they can have access to innovations.
(iii) Enforcement of the WTO regulations will have an effect on local
manufacturing capacity and remove a source of generic innovative quality
drugs on which the poorer countries depend.
(iv) While technology transfer will actually be discouraged, there are
no incentives or provisions to ensure that increased revenues will go
towards the development of essential medical technologies.
Further, while the TRIPS agreement still leaves some loopholes for developing
countries in the form of compulsory licensing and parallel imports,
it is increasingly difficult to choose these options. This is because
the industrial countries - and the US in particular - have been pressurising
several developing countries to implement patent and intellectual property
legislation that is more restrictive than the minimum requirements of
the TRIPS Agreement.
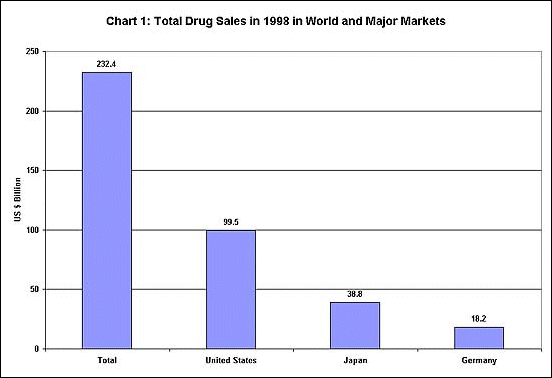
These possibilities become more disturbing given the nature of the international
drug market. As Chart 1 shows, the world market for drugs is a huge
one, but it is dominated by only 3 countries - the United States, Japan
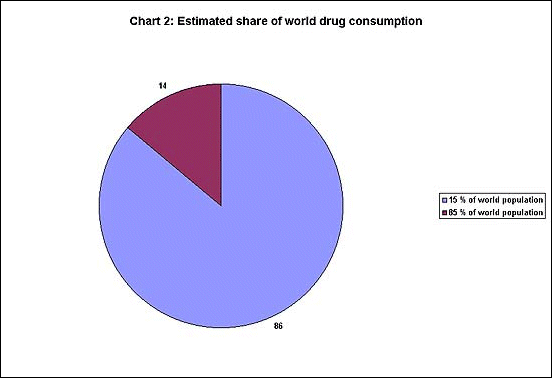
and Germany - which make up more than two-thirds of total sales. Chart
2 shows an even more unequal distribution : only 15 per cent of the
world' population accounts for 86 per cent of drug spending, while the
remaining 85 per cent of the world's population get only 14 per cent
share.
Obviously, this majority is mainly in developing countries. Chart 3 shows the contrast between per capita spending on drugs in several developed and developing countries. Thus Japan shows per capita spending which is several hundred times that of India or Bangladesh.
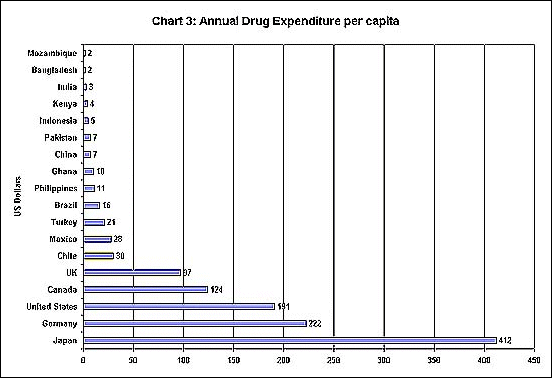
This inequality
helps to understand why research and development for diseases found
in developing countries is not only insufficient, but has almost disappeared
since the 1970s. Between 1975 and 1997, out of 1,223 new chemical entities,
only 13 (1%) were for the treatment of tropical diseases. And of these,
only 4 were the result of R&D activities of the pharmaceutical industry.
This is despite the fact that infectious diseases currently kill 11
million people annually in developing countries, and half of those killed
are children.
The importance of purchasing power in affecting not just the development
of a drug but even its continued production is dramatically illustrated
in the case of eflornithine (Ornidyl) which is a drug to treat sleeping
sickness. This disease, which is transmitted by the tsetse fly, is currently
estimated to kill 150,000 people every year, mainly in Africa. The treatment
was developed by the American firm Merell Dow in 1985, but the price
was so high that it was beyond the reach of those most seriously affected.
Therefore the production of the drug was subsequently abandoned.
The new post-merger owner of the drug, Aventis, finally agreed to transfer
marketing rights to the World Health Organisation (WHO). But the WHO
lacks the resources to manufacture it, and sponsors are still being
sought to finance the production of this drug. By contrast, the fastest
growing segments of world drug production are non-essential drugs such
as Viagra and anti-depressants.
One of the problems with the current international regime is that it
discourages national policies that are designed to regulate drug prices
and drug access. Yet it is now clear that the presence of successful
national drug policies was a major factor in lowering drug prices. This
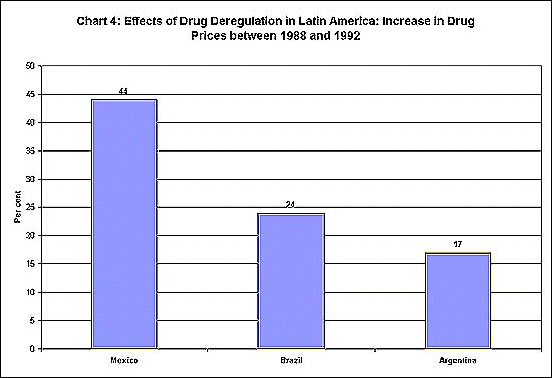
becomes even more clear when such policies are changed. Thus, the enforced
deregulation of domestic drug sectors in Latin America led to a substantial
increase in drug prices in Mexico and Brazil in only 4 years, as Chart
4 shows.
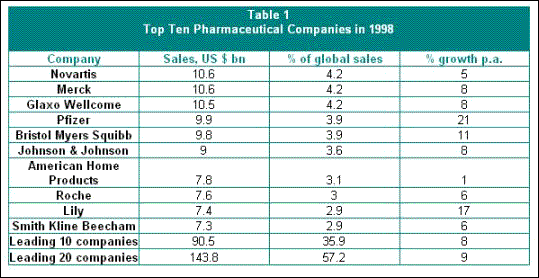
The difficulty of ensuring even a minimum degree of democratic to life-saving drugs is compounded by the high degree of concentration in the international drug industry. Table 1 describes the situation in 1998, when the top ten companies controlled 36 per cent of the market and the top twenty companies controlled 57 per cent of world sales. Since then there have been more mega-mergers which have made the industry even more concentrated. Thus, Glaxo Wellcome has merged with SmithKline Beecham, Pfizer merged with Warner Lambert, and the companies Hoechst-Marion, Merrell and Rhone-Poulenc merged to form Aventis.
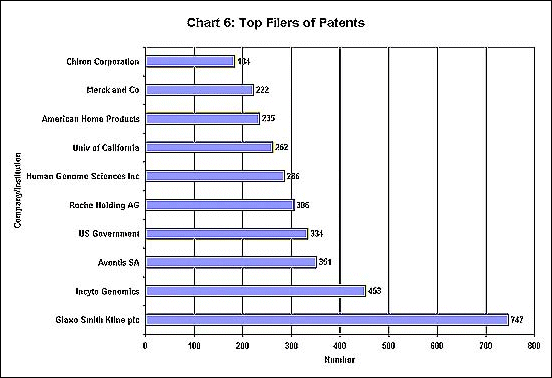
Apart from mergers, there is growing evidence that drug companies are using the patent system to establish monopoly control. Often patents are filed for products or chemical substances or now even genes, whose attributes are not fully known, simply to pre-empt the competition and allow for monopoly rents once further research - possibly by others including public agencies - reveals the uses. As Chart 6 shows, the top ten filers of patents include 6 drug companies and two companies specialising in genetic research.
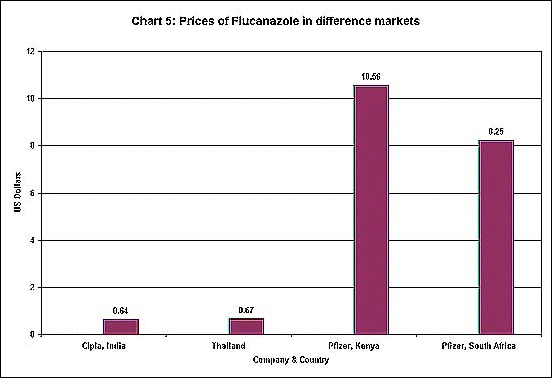
Such monopoly allows
drug companies to charge prices which are as high as they feel the market
will bear, without reference to or well in excess of the actual costs
of R&D that they may have borne. Thus there is wide variation in
prices of the same drug charged not only by different companies but
even by the same company in different markets. This is clear from Chart
5. The drug flucanazole, which is used both for AIDS treatment and for
some forms of meningitis, is available at a substantially lower price
in India and Thailand were generic substitutes are produced. But even
Pfizer, which holds the patent, charges different prices in Kenya and
South Africa.
This use of market segmentation to earn monopoly profits is obviously
constrained by the possibility of undercutting by competitors producing
generic substitutes. This probably underlies the ferocity of the response
of Glaxo to sales of anti-AIDS drugs in Africa by Cipla. After all,
the sales of the drug in Ghana are so small as to be negligible to Glaxo.
However, one representative of the company argued that ".It's the
precedent aspect... The companies are sensitive about a pattern starting
to develop where countries use generics."
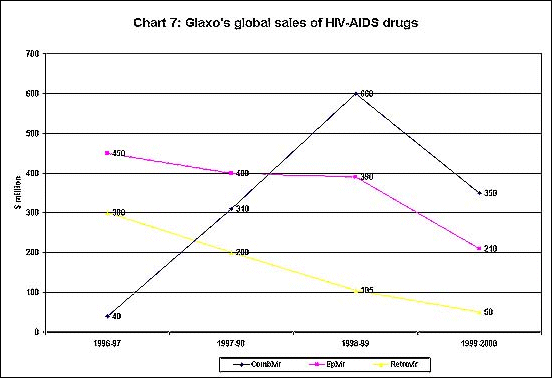
Indeed, this may be a real threat to the monopoly control of the drug
companies. As Chart 7 shows, Glaxo's global sales of its HIV-AIDS drugs
- which are huge money spinners for the company - declined somewhat
in 1999-2000, possibly because of the availability of cheaper generic
substitutes and the consequent compulsion to bring its own prices down
to some extent.
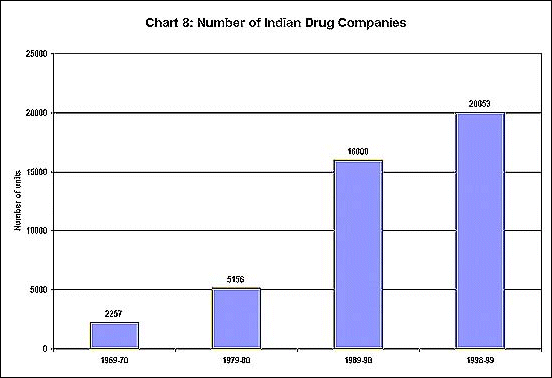
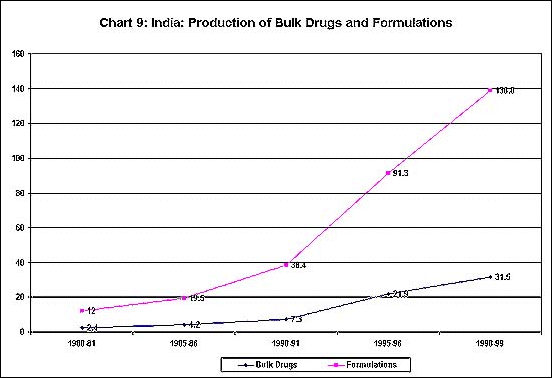
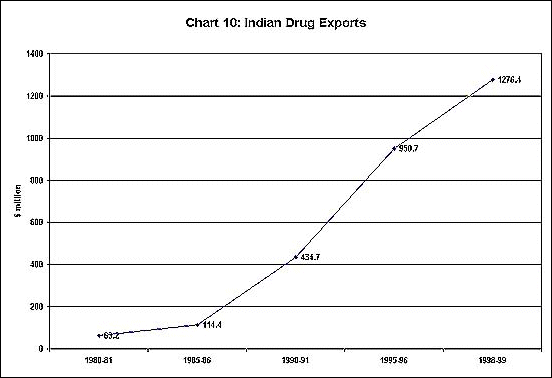
The Indian Patents Act, which allowed for only process patents in the pharmaceutical industry, has played a crucial role in allowing the development and production of generic substitutes through reverse engineering, and has been a basic reason for the dramatic growth of the industry. Not only have the number of units in the industry expanded greatly over three decades (as shown in Chart 8) but the production of both bulk drugs and formulations has been increasing at an impressive rate (Chart 9). Furthermore, exports have also been growing fast, as evident from Chart 10.
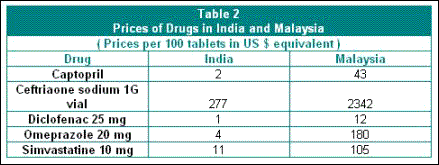
This is because of the major price advantage that Indian companies are able to offer, both because of the ability to engage in reverse engineering and because of the more competitive nature of the domestic industry. This allows for very substantial differences in drug prices between India and other developing countries, even after deregulation which has raised drug prices in India over the past decade. Table 2 gives some indication of the vast variation in drug prices between India and Malaysia, where the patent laws did not allow for the emergence of a vibrant domestic drug industry and where there has been greater reliance on multinationals.
It is now much more widely
recognised that there is no necessary correlation between socially desirable
and necessary R&D in drug development, and a tight patent regime.
Indeed, much of the major research in pharmaceuticals and medicine,
both in the past and currently, is under the aegis of publicly funded
institutions across the world. It is also worth noting that even in
many western countries, pharmaceutical products remained unpatentable
until the 1980s or even the 1990s, with no adverse implications for
research.
It is therefore crucially important to retain the positive and progressive
features of the existing Patents Act, and argue instead for a rethinking
of the severity of the TRIPS agreement especially with respect to drugs
and public health, now that the adverse implications are more widely
known. Instead of rushing to amend our own Patents Act, the Indian government
needs to push for a revision of the TRIPS agreement, for which there
is already much developing country support. It is also important to
support research into drug development which is much more relevant from
the point of view of diseases more widely prevalent in countries like
India, in which area the multinational drug companies have already shown
themselves to be lacking.
© MACROSCAN 2001