|
Apart
from mergers, there is growing evidence that drug companies
are using the patent system to establish monopoly control.
Often patents are filed for products or chemical substances
or now even genes, whose attributes are not fully known,
simply to pre-empt the competition and allow for monopoly
rents once further research - possibly by others including
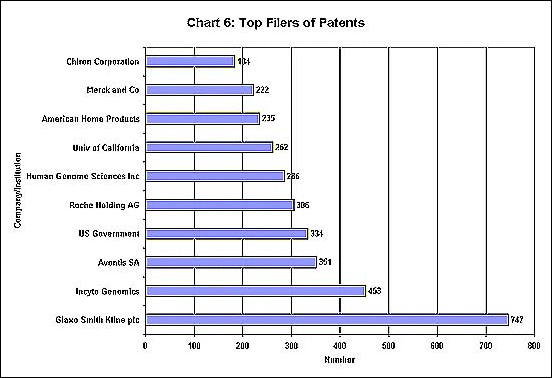
public agencies - reveals the uses. As Chart 6 shows,
the top ten filers of patents include 6 drug companies
and two companies specialising in genetic research.
Chart
5 >> Click
to Enlarge
Chart
6 >> Click
to Enlarge
Such
monopoly allows drug companies to charge prices which
are as high as they feel the market will bear, without
reference to or well in excess of the actual costs of
R&D that they may have borne. Thus there is wide
variation in prices of the same drug charged not only
by different companies but even by the same company
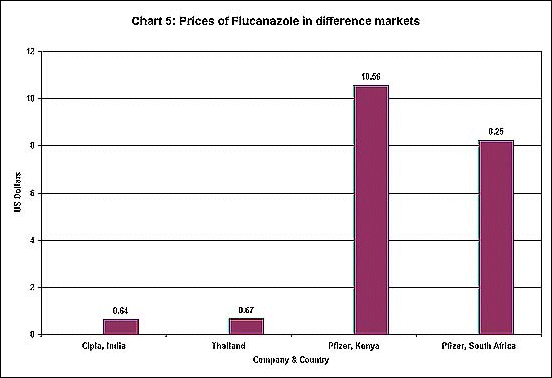
in different markets. This is clear from Chart 5. The
drug flucanazole, which is used both for AIDS treatment
and for some forms of meningitis, is available at a
substantially lower price in India and Thailand were
generic substitutes are produced. But even Pfizer, which
holds the patent, charges different prices in Kenya
and South Africa.
This use of market segmentation to earn monopoly profits
is obviously constrained by the possibility of undercutting
by competitors producing generic substitutes. This probably
underlies the ferocity of the response of Glaxo to sales
of anti-AIDS drugs in Africa by Cipla. After all, the
sales of the drug in Ghana are so small as to be negligible
to Glaxo. However, one representative of the company
argued that ".It's the precedent aspect... The
companies are sensitive about a pattern starting to
develop where countries use generics."
Indeed, this may be a real threat to the monopoly control
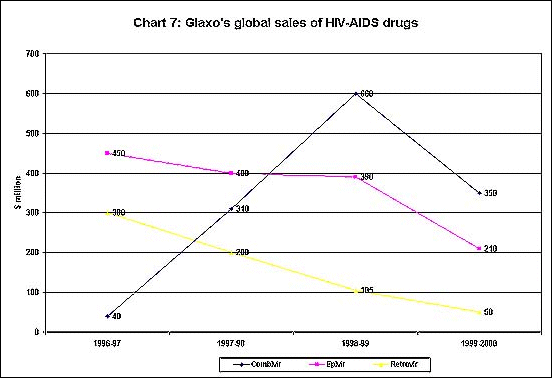
of the drug companies. As Chart 7 shows, Glaxo's global
sales of its HIV-AIDS drugs - which are huge money spinners
for the company - declined somewhat in 1999-2000, possibly
because of the availability of cheaper generic substitutes
and the consequent compulsion to bring its own prices
down to some extent.
Chart
7 >> Click
to Enlarge
The
Indian Patents Act, which allowed for only process patents
in the pharmaceutical industry, has played a crucial
role in allowing the development and production of generic
substitutes through reverse engineering, and has been
a basic reason for the dramatic growth of the industry.
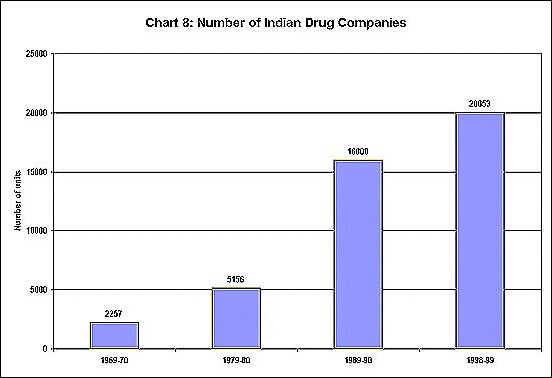
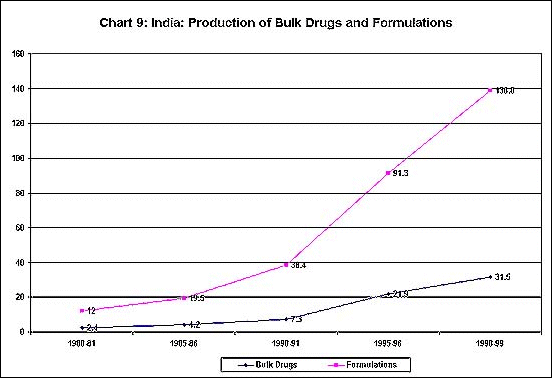
Not only have the number of units in the industry expanded
greatly over three decades (as shown in Chart 8) but
the production of both bulk drugs and formulations has
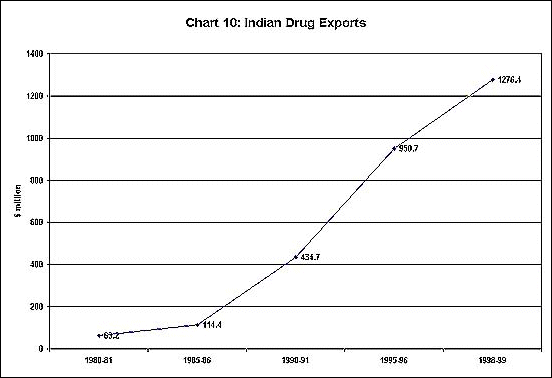
been increasing at an impressive rate (Chart 9). Furthermore,
exports have also been growing fast, as evident from
Chart 10.
Chart
8 >> Click
to Enlarge
Chart
9 >> Click
to Enlarge
Chart
10 >> Click
to Enlarge
This is because of the major price advantage that Indian
companies are able to offer, both because of the ability
to engage in reverse engineering and because of the
more competitive nature of the domestic industry. This
allows for very substantial differences in drug prices
between India and other developing countries, even after
deregulation which has raised drug prices in India over
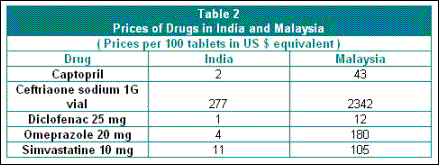
the past decade. Table 2 gives some indication of the
vast variation in drug prices between India and Malaysia,
where the patent laws did not allow for the emergence
of a vibrant domestic drug industry and where there
has been greater reliance on multinationals.
Table
2 >> Click
to Enlarge
It is now much more widely recognised that there is
no necessary correlation between socially desirable
and necessary R&D in drug development, and a tight
patent regime. Indeed, much of the major research in
pharmaceuticals and medicine, both in the past and currently,
is under the aegis of publicly funded institutions across
the world. It is also worth noting that even in many
western countries, pharmaceutical products remained
unpatentable until the 1980s or even the 1990s, with
no adverse implications for research.
It is therefore crucially important to retain the positive
and progressive features of the existing Patents Act,
and argue instead for a rethinking of the severity of
the TRIPS agreement especially with respect to drugs
and public health, now that the adverse implications
are more widely known. Instead of rushing to amend our
own Patents Act, the Indian government needs to push
for a revision of the TRIPS agreement, for which there
is already much developing country support. It is also
important to support research into drug development
which is much more relevant from the point of view of
diseases more widely prevalent in countries like India,
in which area the multinational drug companies have
already shown themselves to be lacking.
|

